RAPID SCREENING OF PHYTOPATHOGENIC Erwinia sp. OF TWO POTATO VARIETIES (SPUNTA AND DESIREE) FROM ALGERIAN AGRICULTURAL FIELDS
Main Article Content
Abstract
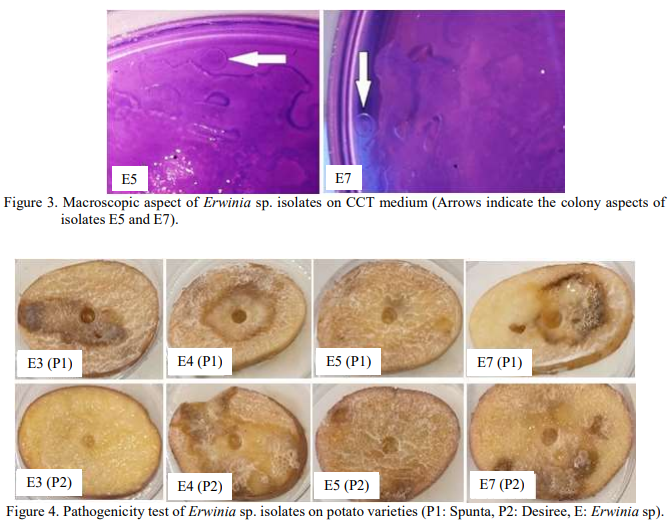
Rapid screening of phytopathogenic Erwinia sp. of two potato varieties (Spunta and Desiree) from Algerian agricultural fileds. Isolation, phenotypic identification and in vitro phytopathogenicity screening of Erwinia sp. from agricultural field of two potato varieties (Spunta and Desiree) in Algeria. The current study aims to isolate, identify and screen phytopathogenic isolates of Erwinia sp. causing potato diseases. The techniques presented in this study for isolation and characterization of phytopathogenic Erwinia sp. are conventional methods that are used in this field of research. Seven phytopathogenic bacteria were recovered from potato tubers of two varieties (Spunta and Desiree). The phenotypic identification allowed characterizing typical colonies of Erwinia sp. on two semi-selective media: King’s B and TCC media. Erwinia sp. formed characteristic colonies on King’s B medium that were round, convex and representing creamy color. While, Erwinia sp. also developed specific colonies on TCC medium which were pale purple, circular, convex, even bulging; smooth and mucous. In vitro phytopathogenicity test on potato slices lead to screen the phytopathogenic isolate E5 characterized by highest rotten tissue zone of (2.33 ± 0.29 cm) and (2.33 ± 0.58 cm) toward Spunta and Desiree varieties, respectively. Followed, by isolate E4 characterized by rotten tissue zone of (1.83 ± 0.58 cm) and (2.17 ± 0.29 cm) toward Spunta and Desiree varieties, respectively; compared to their corresponding uninfected controls. The RTZ (Rotten tissue zone) evidently is proportional to the specific pathogenicity of Erwinia sp. isolates and the characteristic sensitivity of various varieties (Spunta and Desiree). Thus, make determining RTZ a rapid screening technique for the selection of the highest phytopathogenic isolates. This investigation provides valuable information for rapid screening (infected potato tuber) and characterization (isolation using semi-selective media) of pathogenic Erwinia sp. engendering potato disease compared to existing methods like infection of leaves or plants; and phytopathogenic Erwinia sp. identification through PCR amplification or in situ hybridation.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
Acuña I, Mancilla S, Sagredo B, Gutiérrez M, Vargas M, & Delgado J. 2004. Identificación taxonó mica de las especies Fusarium causantes de pudricion seca en tuberculos de papa en el sur de Chile. In: 55º Congreso Agrónomico de Chile, 5º Congreso, Sociedad Chilena de Fruticultura, 1er Congreso Sociedad Chilena de Horticultura.Valdivia, Xª Región–Chile. 19 al 22 de Octubre de 2004.
Aiteche H & Benzid K. 2018. Activité antagoniste de la souche Trichoderma asperellum (T34) sur demi-tubercules de pomme de terre infectés par Dickeya solani. Mémoire de Master, Université de Béjaia.
Allefs JJHM, Van Dooijeweert W, Prummel W, Keizer LCP, & Hoogendoorn J. 1996. Components of partial resistance to potato blackleg caused by pectolytic Erwinia carotovora subsp. atroseptica and E. chrysanthemi. Plant Pathol. 45(3): 486 –496.
Andrivon D, Corbière R, Lucas JM, Pasco C, Gravoueille JM, Pellé R, Dantec JP, & Ellissèche D. 2003. Resistance to late blight and soft rot in six potato progenies and glycoalkaloid contents in the tubers. Am. J. Pot. Res. 80(2): 125–134.
Aremu BR & Babalola OO. 2015. Classification and taxonomy of vegetable macergens. Front Microbiol. 6: 1361.
Azhar M & Wani S. 2021. Wild Germplasm for Genetic Improvement in Crop Plants. Academic Press, London.
Benada M, Boumaaza B, Boudalia S, Khaladi O, & Guessas B. 2018. Variability of aggressiveness and virulence of Erwinia carotovora subsp. carotovorum causing the soft rot on potato tubers in the western of Algeria. Int. J. Plant Biol. 9(1): 52–56.
Benada M. 2019. Caractérisation phénotypique et génotypique d’Ewinia sp. pathogène et essaie de lutte biologique. Thèse. Université Oran 1. Oran.
Borruso L, Salomone-Stagni M, Polsinelli I, Schmitt AO, & Benini S. 2017. Conservation of Erwinia amylovora pathogenicity-relevant genes among Erwinia genomes. Arch. Microbiol. 199(10): 1335–1344.
Boufares K. 2012. Comportement de trois variétés de pommes de terre (Spunta, Désirée et Chubak) entre deux milieux de culture substrat et hydroponique. Thèse de Magistère en Agronomie. Université Aboubekr Belkaid. Tlemcen.
Chehat F. 2008. La filière pomme de terre algérienne, une situation précaire, Journée d’étude sur la filière pomme de terre, situation actuelle et perspectives. E.N.S.A, El-Harrach. 18 Juin 2008. pp.1–13.
CIPV. 2016. NIMP 27: Protocoles de diagnostic pour les organismes nuisibles réglementés. PD 13: Erwinia amylovora. https://www.ippc.int/static/media/files/publication/fr/2017/02/DP_13_2016_Fr_2017-01-11.pdf. Accessed on 17 June 2021.
Czajkowski R, Pérombelon MCM, van Veen JA, & van der Wolf JM. 2011. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant Pathol. 60(6): 999–1013.
Daoud H & Doudou O. 2017. Etude comparative de 14 variétés de pomme de terre cultivée (Solanum tuberosum L.) dans la région de Mostaganem. Mémoire de Master en Agronomie. Université Abdelhamid Ibn Badis, Mostaganem.
De La Fuente L & Burdman S. 2011. Pathogenic and beneficial plant associated bacteria. In: Lal R (Ed.). Agricultural Science. EOLSS Publishers, Oxford.
Eduardo LG, Ramirez BS, Maribel CF, Pescador MGN, & Cruz FJM. 2018. Low accuracy of the McFarland method for estimation of bacterial populations. Afr. J. Microbiol. Res. 12(31): 736–740.
Elhalag K, Elbadry N, Farag S, Hagag M, & Hussien A. 2020. Etiology of potato soft rot and blackleg diseases complex in Egypt. J. Plant Dis. Prot. 127: 855–871.
FAOSTAT. 2015. Food and Agriculture Organization of the United Nations. Produit par pays. Available via. http://www.fao.org/faostat/en/#home. Accessed on 17 June 2021.
Foudil FZ. 2016. La première variété de pomme de terre algérienne portera le nom de Omnia. https://www.elwatan.com/pages-hebdo/etudiant/la-premiere-variete-de-pomme-de-terre-algerienne-portera-le-nom-de-omnia-16-03-2016. Accessed on 16 June 2021.
Ginzberg I, Tokuhisa JG, & Veilleux RE. 2009. Potato steroidal glycoalkaloids: biosynthesis and genetic manipulation. Potato Res. 52(1): 1–15.
Hajimehdipoor H, Samadi N, Mozaffarian V, Rahimifard N, Shoeibi S, & Pirali HM. 2010. Chemical composition and antimicrobial activity of Oliveria decumbens volatile oil from west of Iran. J. Med. Plants. 9(6): 39–44.
Hamad Y, Shaaban WI, Youssef SAG, & Balabel NM. 2021. Varietal differences and their relation to brown rot disease resistance in potato. In: Awaad H, Abu-hashim M, & Negm A (Eds.). Mitigating Environmental Stresses for Agricultural Sustainability in Egypt. pp. 251–269. Springer Nature, Switzerland.
Hélias V, Andrivon D, & Jouan B. 2000a. Development of symptoms caused by Erwinia carotovora ssp. atroseptica under field conditions and their effects on the yield of individual potato plants. Plant Pathol. 49(1): 23–32.
Hélias V, Andrivon D, & Jouan B. 2000b. Internal colonization pathways of potato plants by Erwinia carotovor ssp. atroseptica. Plant Pathol. 49(1): 33–42.
Holt JG, Bergey DH, & Krieg NR. 1986. Bergey’s Manual of Systematic Bacteriology, Vol. 1 & 2. Williams & Wilkins, Baltimore.
Ibrahim M, Jouan, B, Samson R, Poutier F, & Saily M 1978. Prospect of a pathogenicity test concerning Erwinia carotovora var. atroseptica and Erwinia carotovora var. carotovora on half potato tubers, variation according to variety of bacterial species and stains, inoculums dose, temperature, variety of potatoes, physiological age of tubers and delay between injury and inoculation. Proceeding 4th International Plant Conference plant pathogenic Bacteria. pp. 591–602. Angers-França: INRA, Angers. France.
Kado CI. 2006. Erwinia and related genera. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (Eds.). The Prokaryotes. pp. 443–450. Springer, New York.
Kheddam H, Allal-Benfekih L, Bendifallah L, & Moudoud R. 2017. Effet de la variation climatique des zones de production sur le comportement et le rendement de variétés introduites de pomme de terre en Algérie. Agrobiologia. 7(2): 635–643.
Koepsell PA. 1978. Controlling bacterial soft rot and blackleg of potatoes. https://ir.library.oregonstate.edu/downloads/db78tc24x. Accessed on November 2020.
Kudela V. 2009. Potential impact of climate change on geographic distribution of plant pathogenic bacteria in central Europe. Plant Prot. Sci. 45: S27–S32.
Ladjouzi R. 2006. Recherche et identification des Pectobacterium, agents de la pourriture molle sur différentes plantes hôtes: pommes de terre, tomates et carottes. Mémoire de Magister. Université Abderrahmane Mira. Béjaia.
Lacroix C & Vézina L. 2003. Technique de laboratoire pour le diagnostic des bactéries phytopathogène. Laboratoire de diagnostic en phytoprotection pour la détection et l’identification des bactéries phytopathogènes. Mémoire DEA . Université de Rennes I. Rennes.
Lakhdar F. 2018. Contribution à l’étude des potentialités antiproliférative et antibactérienne des algues brunes et rouges de la côte d’El Jadida pour une valorisation médicale et environnementale. Thése. Université de Nantes. Nantes.
Latour X, Faure D, Diallo S, Cirou A, Smadja B, Dessaux Y, & Orange N. 2008. Lutte contre les maladies bactériennes de la pomme de terre dues aux Pectobacterium spp. (Erwinia carotovora). Cah. Agric. 17(4): 355–360.
Lelliott RA & Dickey R. 1984. Genus VII. Erwinia. In: Krieg NR & Holt JG (Eds.). Bergey’s Manual of Systematic Bacteriology, Vol. 1. pp. 469–476. The Williams & Wilkins Co, Baltimore.
LNPV. 2005. Mise en évidence d’ Erwinia amylovora à partir de végétal symptomatique par isolement et identification de la souche. Laboratorie National de la Protection des Végétaux, Unité Bactériologie.
Markoviæ S, Stankoviæ S, Jelušiæ A, Iliciæ R, Kosovac A, Poštiæ D, & Popoviæ T. 2021. Occurrence and identification of Pectobacterium carotovorum subsp. brasiliensis and Dickeya dianthicola causing blackleg in some potato fields in Serbia. Plant Dis. 105(4): 1080–1090.
Moh AA, Massart S, Jijakli MH, & Lepoivre P. 2012. Models to predict the combined effects of temperature and relative humidity on Pectobacterium atrosepticum and Pectobacterium carotovorum subsp. carotovorum population density and soft rot disease development at the surface of wounded potato tubers. J. Plant Pathol. 94(1): 181–191.
Nabhan S, De Boer SH, Maiss E, & Wydra K. 2013. Pectobacterium aroidearum sp. nov., a soft rot pathogen with preference for monocotyledonous plants. Int. J. Syst. Evol. Microbiol. 63(7): 2520–2525.
Pédron J, Schaerer S, Kellenberger I, & Van Gijsegem F. 2021. Early emergence of Dickeya solani revealed by analysis of Dickeya diversity of potato blackleg and soft rot causing pathogens in Switzerland. Microorganisms. 9(6): 1187.
Pérombelon MCM & Kelman A. 1980. Ecology of the soft rot erwinias. Ann. Rev. Phytopathol. 18: 361-387.
Pérombelon MCM. 1994. Diversity in erwinias as plant pathogens. In: Lemattre M, Freigoun, S, Rudolph K, & Swings JG (Eds.). Plant Pathogenic Bacteria. pp. 113–128. Versailles, France. Les Colloques 66, INRA-ORSTOM, Paris.
Pérombelon MCM & van der Wolf JM. 2002. Methods for the detection and quantification of Erwinia carotovora subsp. atroseptica (Pectobacterium carotovorum subsp. atrosepticum) on potatoes: A laboratory manual. Scottish Crop Research Institute, Scotland.
Priou S & Jouan B. 1992. Comparison of the effectiveness of two methods of screening potato to solf rot induced by Erwinia carotovora ssp. atroseptica. Proceeding of the joint conference of the EAPR breeding and varietal assessment section and the EUCARPIA potato section. pp. 139–140. Landerneau, France.
Priou S & Jouan B. 1996. Les maladies provoquées par les bactéries pathogènes du genre Erwinia. In: Rousselle P, Robert Y, & Crosnier JC (Eds.). Mieux Comprendre La Pomme de Terre. pp. 260–265. INRA Editions, Paris.
Pritchard L, Humphris S, Saddler GS, Parkinson NM, Bertrand V, Elphinstone JG, & Toth IK. 2012. Detection of phytopathogens of the genus Dickeya using a PCR primer prediction pipeline for draft bacterial genome sequences. Plant Pathol. 62(3): 587–596.
Rahman MM, Ali ME, Khan AA, Hashim U, Akanda AM, & Hakim MA. 2012. Characterization and identification of soft rot bacterial pathogens in Bangladeshi potatoes. Afr. J. Microbiol Res. 6(7): 1437–1445.
Raimi A, Adeleke R, & Roopnarain A. 2017. Soil fertility challenges and biofertiliser as a viable alternative for increasing smallholder farmer crop productivity in sub-Saharan Africa. Cogent Food Agric. 3(1): 1400933.
Rabot B, Pasco C, & Schmidt J. 1994. Assessing six Austrian potato cultivars for resistance to Erwinia carotovora subsp. atroseptica. Potato Res. 37(2): 197–203.
Rienzie R, Sendanayake L, De Costa D, Hossain A, Brestic M, Skalicky M, Vachova P, & Adassooriya NM. 2021. Assessing the carboxymethylcellulose copper-montmorillonite nanocomposite for controlling the infection of Erwinia carotovora in potato (Solanum tuberosum L.). Nanomaterials. 11(3): 802.
Rosenzweig N, Steere L, Hammerschmidt R, & Kirk W. 2016. Tuber soft rot, blackleg and aerial stem rot. Extension Bulletin E-3335: 1–4.
Salem EA & Abd El-Shafea YM. 2018. Biological control of potato soft rot caused by Erwinia carotovora subsp. carotovora. Egypt J. Biol. Pest Control. 28: 94.
Smadja B, Latour X, Trigui S, Burini JF, Chevalier S, & Orange N. 2004. Thermodependence of growth and enzymatic activities implicated in pathogenicity of two Erwinia carotovora subspecies (Pectobacterium spp.). Can. J. Microbiol. 50(1): 19–27.
Stevenson WR, Loria R, Franc GD, & Weingartner DP. 2001. Compendium of Potato Diseases. The American Phytopathological Society. Saint Paul, Minnesota.
Toth IK, Bell KS, Holeva MC, & Birch PRJ. 2003. Soft rot erwiniae: from genes to genomes. Mol. Plant Pathol. 4(1): 17–30.
van der Wolf JM & De Boer SH. 2007. Bacterial pathogens of potato. In: Vreugdenhil D, Bradshaw J, Gebhardt C, Govers F, Taylor M, MacKerron D, & Ross H (Eds.). Potato biology and biotechnology: Advances and Perspectives.1st edition. pp. 595–617. Elsevier Delft, Netherlands.
Waleron M, Waleron K, & Lojkowska E. 2004. Characteristics, identification, differentiation and taxonomy of plant pathogenic bacteria from the genus Erwinia. Postepy. Mikrobiolii. 43(3): 297–319.
Yahiaoui-Zaidi R, Jouan B, & Andrivon D. 2003. Biochemical and molecular diversity among Erwinia isolates from potato in Algeria. Plant Pathol. 52(1): 28–40.
Yap MN, Barak JD, & Charkowski AO. 2004. Genomic diversity of Erwinia carotovora subsp. carotovora and its correlation with virulence. Appl. Environ. Microbiol. 70(5): 3013–3023.